The effective provision of medical device field service is not a simple process to optimize. There are many factors that influence operations, from resources and technology to staff management and coordination. Field service organizations that keep track of all of these different factors manage to provide a seamless and efficient service and keep their customers satisfied.
But no business is perfect from the get-go. In most cases, a period of trial and error is unavoidable while getting a grasp of the intricacies of running and managing field service operations, especially when it comes to medical devices. Being aware of the most common mistakes made in the delivery of field service support can significantly shorten the learning curve.
With that in mind, here are some of the most common errors that medical device field service organizations commit while providing and managing their service:



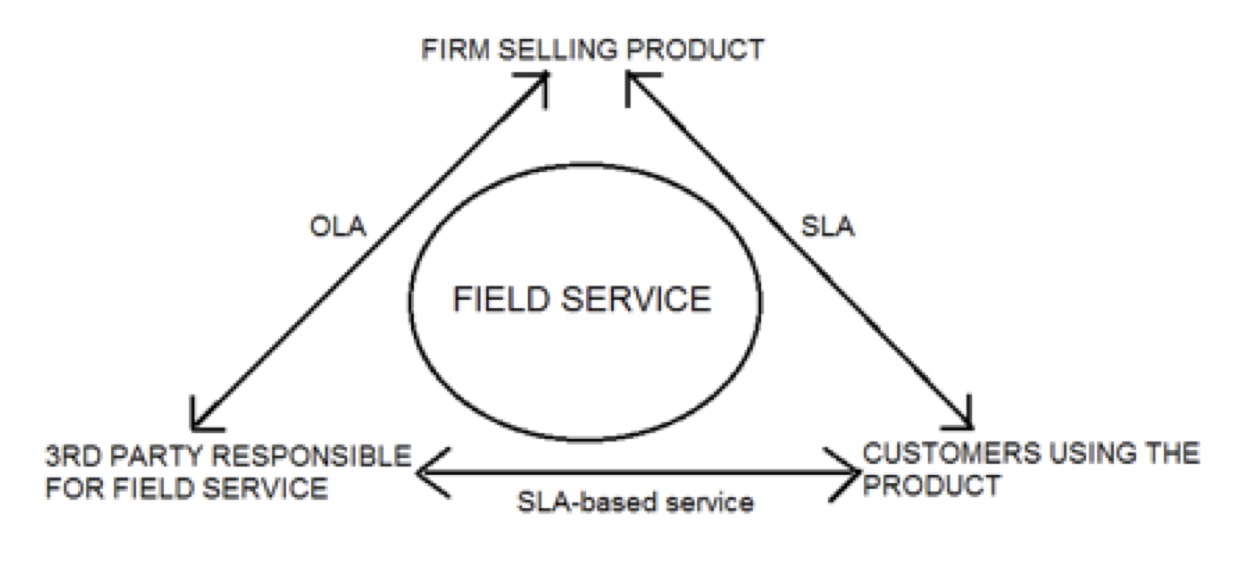

 Bringing a new medical device to the market is a Herculean task. It requires all of the standard start up work such as determining product market fit, product design, manufacturing and production, marketing message, and much more. In addition to these, since it is a medical device it requires layers and layers of FDA approval, which is akin to hiking up a mountain carrying an 800-pound backpack.
Bringing a new medical device to the market is a Herculean task. It requires all of the standard start up work such as determining product market fit, product design, manufacturing and production, marketing message, and much more. In addition to these, since it is a medical device it requires layers and layers of FDA approval, which is akin to hiking up a mountain carrying an 800-pound backpack.


 I am the CEO of a service provider that provides diagnostic testing services to over 300 medical practices across the country. Our clientele ranges from the sole practitioner to extensive multi-specialty practices, to everything in-between. I am also the former Chief Marketing Officer for a top digital marketing agency in Chicago.
I am the CEO of a service provider that provides diagnostic testing services to over 300 medical practices across the country. Our clientele ranges from the sole practitioner to extensive multi-specialty practices, to everything in-between. I am also the former Chief Marketing Officer for a top digital marketing agency in Chicago. 
